In response to a question from a U.S. children’s rights protection group, the Udairai Foundation, Dogra’s director admitted that the hospital had conducted clinical drug trials on 4142 infants since January 2006. Of these, 2,728 infants were under the age of 1,49 The infant died in drug trials and "at least five well-known Western pharmaceutical companies conduct infant drug trials in the hospital."
Uday Foundation spokesman Verma said: "Many infant parents complained that their children were only minor problems, but after being sent to the All-Institute of Medical Sciences, they were often told that they needed to be hospitalized for a long time, and many infants would not have the opportunity to leave the hospital again. â€
India is a country where the past, present and future coexist. The proportion of illiterate adults is as high as 39%. Nearly 40% of households still use kerosene lamps. Verma said that the parents of many babies are not well-educated and are easily misled and deceived by so-called professionals. According to the author's understanding, since the All India Medical Academy is a completely free public medical institution, there are indeed many poor people and even illiterate people there. It is not easy for parents of children to clearly understand the consequences of infant drug tests.
This scandal shocked all walks of life in India and people fear that mercenary Western drug companies will use some Indian medical institutions that lack medical ethics to "take shortcuts." India’s ruling Congress Party also issued a statement: “This practice of using infants as experimental products to test drugs must be stopped.â€
However, western pharmaceutical companies and Indian medical institutions have formed a complex interest community in India. It is easier for vulnerable Indian patients to maintain their own interests.
The Indian State Planning Commission official told the author that India has become one of the major human clinical trial sites for Western pharmaceutical companies in Asia. Currently, foreign pharmaceutical companies, mainly Western pharmaceutical companies, are conducting human trials of 139 drugs in India. The reason why these Western companies particularly favor the outsourcing of clinical trials to Indian medical institutions is mainly due to the complexity of the Indian population gene pool and the low cost of such experiments.
India has a population of 1.1 billion and there are enough test subjects. Ethnically speaking, India has whites, yellows, and blacks, and it is an ideal large gene pool that facilitates new drug testing. Many people in India have never used any medicine to treat diseases because of poverty. This will avoid interference between new drugs and old drugs.
More importantly, the cost of clinical trials in India is much lower. For example, recruiting the same level of scientific research personnel in India, the reward is at least half lower than that in Europe and the United States; at the same time, the compensation to Indian subjects and compensation for accidents are very low. According to estimates by the Netherlands Agricultural Cooperation Bank, conducting clinical trials in India can reduce research and development costs by 60%. Some well-known western “think tanks†have also lobbied the Indian government to “provide clinical drug trials as new outsourcing stars like the IT industryâ€. These Western "think tank" institutions have even drawn a "big multibillion-dollar US dollars in human clinical trial outsourcing business in India by 2010".
According to the author's investigation, many medical institutions, including some countries in India and Africa, are actively competing for "orders" for clinical drug trials by Western pharmaceutical companies.
Officials of the Indian State Planning Commission also revealed that the number of personnel currently responsible for the control of human clinical trials in India is as low as 40,000, including pilot survey experts, audit experts, ethic experts and data security management experts. Even the drug trials are responsible for approval. The staff of the Drug Control Department are all seriously deficient. Due to the weak supervision, Western pharmaceutical companies and domestic clinical trial institutions have often taken consideration of interests and tried to obtain test results as soon as possible, resulting in various violations and violations. The All India Academy of Medical Sciences that caused this scandal also had to admit that it is not until now that the hospital has not figured out how many infants who died from the use of experimental drugs, and how many of them belong to the control group. In Western countries, the general principle of human trials is that medical institutions must strictly perform the informed consent procedure and cannot use unfair means such as concealment, deception, inducement, and coercion. Patients have the right to understand the full contents of the diagnosis and treatment process, including possible risks and benefits. The result, the required cost, etc., can also be completely rejected. Because of this, in the United States, only 3% of cancer patients are willing to participate in clinical trials of new drugs. In India, 90% of drug users are helpless poor people. After listening to western pharmaceutical companies and India's domestic "experts" to stir up insults, what can they do in addition to eventually becoming a "white rat" in the interests of the western drug makers and Indian clinical trial institutions? What about other options?
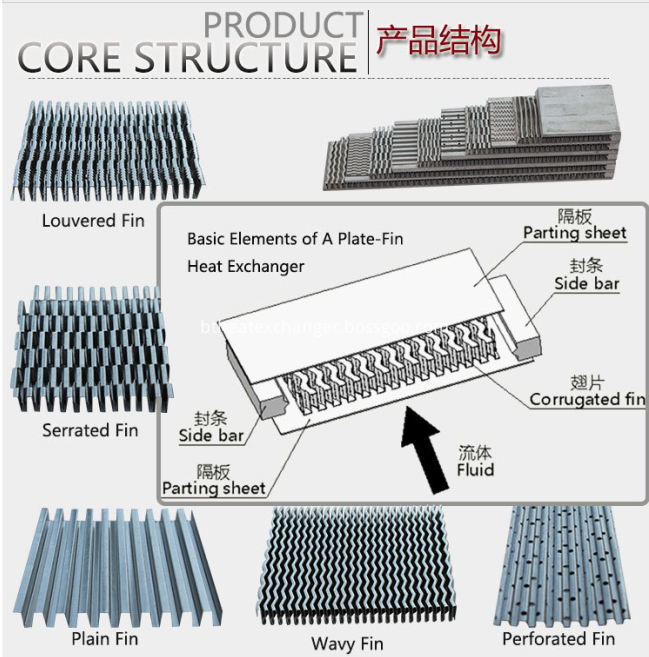
Aluminum Coolers for Compressor, Aluminum plate&bar heat exchangers (oil cooler, air cooler/charge air coolers,compressor coolers, water cooler and combi coolers) have been widely applied for all kinds of compressor including air compressors, LNG-BOG recovery compressor, CNG compressor and more.
Heat Exchanger for Air Dryer, The heat exchangers transfer heat between a cold medium and the damp compressed air. This cools the compressed air to a pressure dew point. If required the condensation generated by the cooling process is collected in a condensation chamber integrated into the heat exchanger and continually drained off.
Advantage:
Compact, Lightweight, High Efficient Heat Transfer

Customize High Quality Aluminum plate-fin cooler upon your drawing, sample or parameter requirement.

FAQ
Q1:Could you customize oil cooler/heat exchanger?
We can manufacture plate-fin heat exchanger/oil cooler /intercooler according to your drawings or samples or parameter requirement.
Q2: I want to buy your products, how can I pay?
A :You can pay via T/T
Q3: How can you guarantee the quality?
A: One year's warranty against B/L date.
Q4: If we don`t find what we want on your website, what should we do?
A: You can email us the descriptions and pictures of the products you need, We will check whether we can make it.
Q5: Can we buy samples of each item for quality testing?
A: Yes, we understand quality test is important and we are glad to send the sample for quality testing.
Q6: What is the lead time?
A: Normal : Plate-fin heat exchanger for 2~3 weeks; Tube&fin or stacked layer coolers for 5 weeks. Exact time upon negotiation
Air Compressor Oil Cooler,CNG Compressor Aluminum Cooler,Compressor Gas Cooler and Radiator Assembly,Compressor Coolers,Charge Air Coolers
Wuxi Better Technology Co., Ltd , https://www.btheatexchanger.com